NOBEL PRIZE 2019 FOR CHEMISTRY
10, Oct 2019
Prelims level : Science & Technology
Mains level : GS-III- Science and Technology - Developments and their applications and effects in Everyday Life; Awareness in the fields of IT, Space, Computers, Robotics, Nano-Technology, Bio-Technology.
Why in News?
- The 2019 Nobel Prize in Chemistry has been announced and the awardees were as John B Goodenough, M Stanley Whittingham and Akira Yoshino for the development of lithium-ion batteries.
About the Award:
- The Nobel Prize in Chemistry is awarded annually by the Royal Swedish Academy of Sciences to scientists in the various fields of chemistry. It was first awarded in 1901.
Evolution of Lithium Ion Batteries:
- These three scientists created the right conditions for a wireless and fossil fuel-free society. The foundation of the lithium-ion batteries was laid by Stanley Whittingham during the oil crisis of 1970’s.
1.Stanley Whittingham
- He started developing methods that could lead to fossil fuel-free energy technologies.
- In the 1970’s, he harnessed the huge tendency of lithium-the lightest metal to give away it’s electrons to make a battery capable of generating just over two volts.
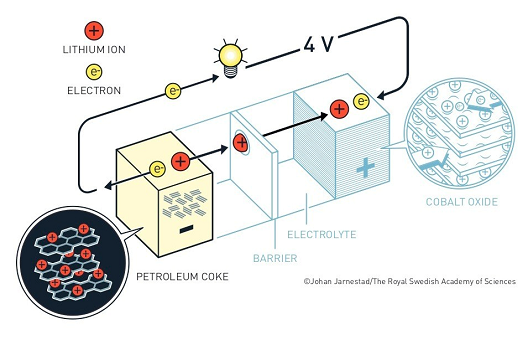
- He used titanium disulphide as cathode and lithium, which is highly reactive, as anode.
2.John B Goodenough
- In the 1980’s he replaced titanium disulphide with cobalt oxide as the cathode. He demonstrated that cobalt oxide with intercalated lithium ions can produce as much as four volts.
- The battery’s potential doubled because of oxide in the cathode but the use of reactive lithium remained a concern.It remained too explosive for general commercial use.
- Goodenough,who is considered an intellectual giant of solid-state chemistry and physics, is the oldest person (97) to ever win a Nobel Prize.
3.Japanese Chemist Akira Yoshino
- He replaced lithium with petroleum coke, a carbon material, which drew the Li-ions towards it. Once the battery was operational, the ions and electrons flowed towards the cobalt oxide cathode.
- The result was a lightweight, hardwearing battery that could be charged hundreds of times before its performance deteriorated.
- The advantage of lithium-ion batteries is that they are not based upon chemical reactions that break down the electrodes, but upon lithium ions flowing back and forth between the anode and cathode.
About Li-Ion battery:
- Lithium-ion battery is type of rechargeable battery that contains several cells. Each cell consists of cathode, anode and electrolyte, separator between electrodes and current collectors. In it, lithium ions move from negative electrode to positive electrode during discharge and back when charging. Li-ion battery use intercalated lithium compound as one electrode material.
Benefits of Li-Ion battery:
- It is light weighted and is one-third the weight of lead acid batteries. It is nearly 100% efficient in both charging and discharging as compared to lead battery which has 70% efficiency.
- It completely discharges i.e. 100% as compared to 80% for lead acid. It has life cycle of 5000 times or more compared to just 400-500 cycles in lead acid.
- It also maintains constant voltage throughout entire discharge cycle whereas voltage in lead acid battery drops consistently throughout its discharge cycle.
- It is much cleaner technology and is safer for environment as it does not have environmental impact as lead acid battery. It can power any electrical application without the need of physical wires-means wireless.






