Mendeleev and his Periodic Table of Elements
18, Jun 2019
Prelims level : Science & Technology
Mains level : GS-III: Technology
The Modern Periodic Table:
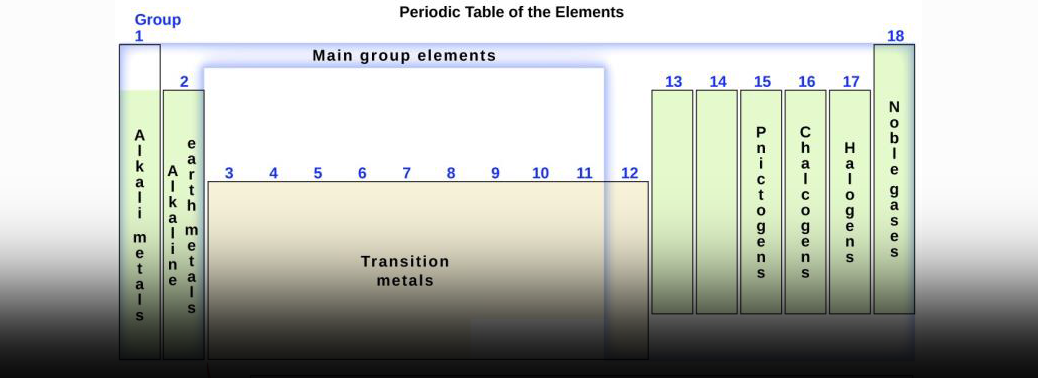
- The periodic table is an arrangement of all the elements known to man in accordance with their increasing atomic number and recurring chemical properties.
- They are assorted in a tabular arrangement wherein a row is a period and a column is a group. Until 1863, the world was aware of only 56 known elements.
- The rate of scientific progress was such that every year, a new element was being discovered. It was during this time that Mendeleev came up with the idea of the Periodic Table.
- He published the Periodic Table in his book– The Relation between the Properties and Atomic Weights of the Elements.
- Mendeleev said that he arrived at the idea in his dream, where he saw all chemical elements falling into place on a table according to their chemical properties.
- Mendeleev had found a definitive pattern following which, each element could be placed according to their atomic weight.
- He had also predicted the qualities of the ‘missing’ (yet to be discovered) elements and gave them Sanskrit names.
Evolution of the Table:
- The noble gases including helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn) were added to the table between 1895 and 1901.
- Likewise, additions have been made to the periodic table as new elements have been discovered in the last hundred years.
- In 1914, English physicist Henry Gwyn-Jeffries Moseley found out that each atomic nucleus can be assigned a number, according to the number of protons in that atom. This changed the way the periodic table worked. The table was redesigned according to the atomic number of elements rather than their atomic weight. Rare-earth elements, including the elements in the Lanthanide series, were included in the atomic table in the late 19th century.






